Can Large Automated Equipment Be Safely Integrated into a Biosafety Cabinet?

Placing large-sized automated equipment inside a Biosafety Cabinet (BSC) is generally not recommended, as it can obstruct airflow, compromise sterility, and threaten research integrity. However, as laboratories increasingly adopt robotic systems, a critical question arises: what if the samples processed by automated equipment are sensitive materials that require biosafety protection?
Why Does Automation Equipment Need a Biosafety Cabinet?

Many laboratories now process sensitive biological samples using
automated systems such as robotic liquid handlers, flow
cytometers, cell sorters, and bioprinters. These systems offer
advantages in speed, precision, consistency, and data quality.
However, when handling primary cells, viruses, genetically
modified organisms, or patient-derived materials, additional
protection is required to safeguard operators, samples, and the
environment.
Automated equipment is usually made for standard laboratory
benches. These benches do not offer enough protection against
cross-contamination, aerosol generation, or accidental exposure.
Some systems have internal filters. However, these filters only
protect the samples inside the instrument; they do not protect
operators or the surrounding environment.
For this reason, integrating automated equipment with a Class II Biosafety Cabinet becomes necessary to ensure operator protection, product sterility, and environmental safety in accordance with ISO Class 5 airflow standards and applicable regulations.
The Challenge of Integrating Automation into
a Biosafety Cabinet

Once the need for biosafety protection is addressed, the next
challenge is integration. Biosafety Cabinets are generally
designed in sizes ranging from 3–6 ft and are intended for
manual work performed by operators. In contrast, automated
systems such as liquid handling platforms and flow cytometers
often exceed the height, width, and weight limitations of a
standard BSC.
Even when equipment can physically fit inside a cabinet, its
presence may disrupt unidirectional airflow, compromise
sterility, and reduce overall safety. Placing large objects
inside a standard BSC is therefore strongly discouraged, as it
undermines the cabinet's primary function.
In addition to airflow concerns, automated systems may introduce
vibration, heat generation, and increased electrical and data
connectivity requirements.
If not properly managed, these factors can interfere with both equipment performance and biosafety conditions, as well as hinder laboratory workflows.
How Esco Integrated Automated Equipment into
the Biosafety Cabinet
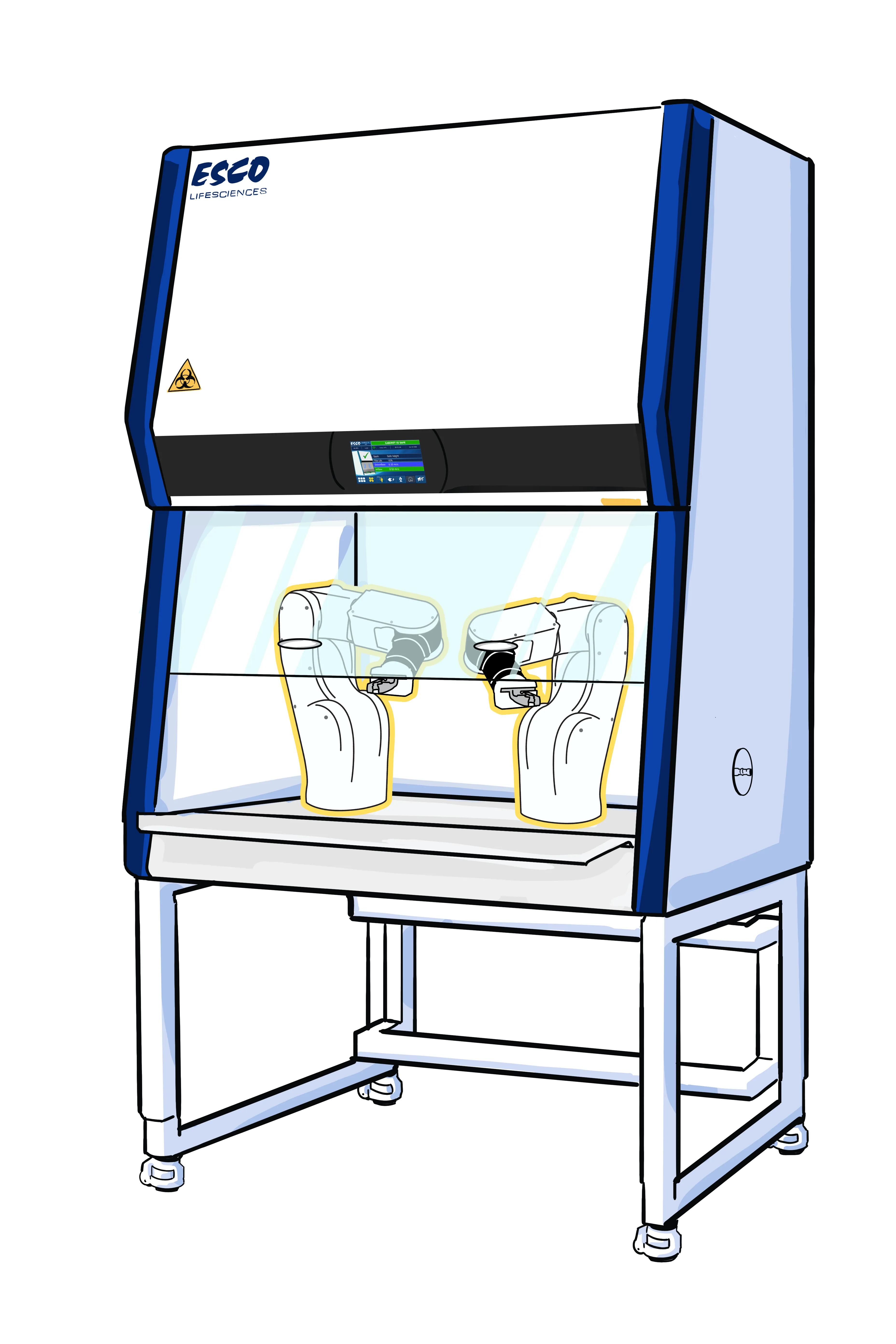
At Esco, these challenges are addressed through a customized
engineering approach. Through in-depth discussions between
customers and Esco engineers, leads to a custom Class II BSC is
designed to safely fit automated equipment while ensuring proper
airflow and containment performance.
The customization extends beyond cabinet size. Internal and
external dimensions are adjusted to support equipment weight and
layout, while additional technical features are incorporated to
support stable operation. These may include vibration dampers,
thermal management, secure cable routers, and internal
connectivity solutions. This setup creates a controlled
environment for automated systems in operation.
Each customized BSC undergoes testing and validation to ensure performance meets customer requirements and applicable standards. This approach has been successfully implemented and validated for automated liquid handling systems and flow cytometers.
Esco’s BSCs offer fully customizable designs that fit existing laboratory spaces while maintaining their primary function of protecting users, products, and the environment. Well-designed automation integration is not merely an expanded workspace, but a purpose-built system that combines automation efficiency with fundamental biosafety protection.
To support this complex integration, Esco’s global service network and experienced technical team assist with installation and validation, to ongoing support. We would be pleased to support your project from conception through validation as you integrate automated equipment into a safe and sterile laboratory workflow.