CAR T-Cell Therapy: A Breakthrough in Fighting Cancer Cells
Therapeutic advances targeting cancer cells are on the rise. However, some cancer therapies have certain limitations due to varying responses among patient and have high relapse rate with poor prognosis. Overcoming these limitations proved to be a major challenge. With few or no available treatment in eradicating malignant cells, a greater understanding of the immune system can be used to improve and lessen the side effects of existing treatment for patients with blood cancers. Recently, a new form of treatment was made available in which the immune cells or antibodies can recognize and kill cancer cells. This treatment is known as CAR T-cell therapy.
What is CAR T-cell therapy?
Chimeric antigen receptor (CAR) T-cell therapy uses genetic engineering to help patients’ own T-cells to recognize and attack the cancer cells without affecting the neighboring healthy cells.
Use of CAR T-cells as a treatment for cancer has been most extensively investigated in patients with B cell malignancies, and early results have been encouraging. The development of CAR T-cell therapy has now expanded beyond phase 1 trials and moved into phase 2 multi-site trials.
How CAR-T Cell Therapy Works?
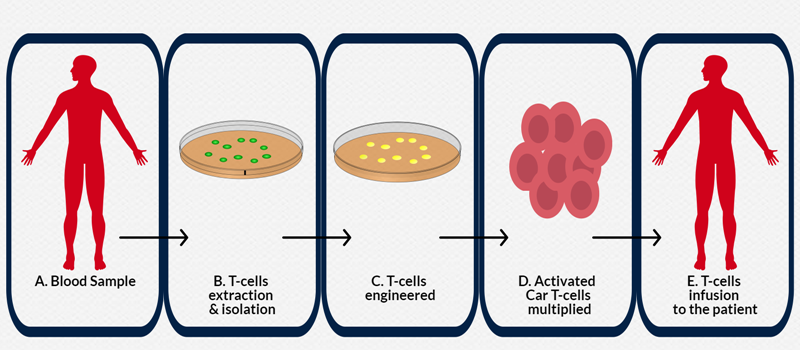
Figure 1. Manufacturing and delivery of pipeline of CAR T-cell Therapies. (A) Peripheral blood mononuclear cells are harvested from T-cell donor then the remaining blood will be returned back to the body. (B) T-cells are genetically re-engineered in the laboratory to produce chimeric antigen receptors (CARs) on their surface. (C) The genetically re-engineered T-cells are known as chimeric antigen receptor (CAR) T-cells. (D) The number of the patient’s genetically modified T-cells is “expanded” by growing cells in the laboratory until there are many millions of them. (E) The patient undergoes infusion of the CAR T-cells.
Results & Future of CAR T-Cell Therapy
Having exhausted all possible treatments, 63 pediatric patients with acute lymphoblastic leukemia have undergone phase 2 trial by Novartis. Out of the 63 patients, 83 percent or 52 children achieved remission. Due to the large percentage of success, the panel unanimously voted 10-0 for the approval of the treatment However, further research needs to be done. Patients undergoing this therapy will be observed for 15 years to check the efficacy of the treatment.
* Novartis is a registered trademark owned by Novartis International AG.
References:
[1]Blaser, K. (2016). CAR T Cell Therapy: A Game Changer in Cancer Treatment. Hindawi Publishing Corporation: Journal of Immunology Research, Volume (2016), 1-5.
[2]Chimeric Antigen Receptor (CAR) T-Cell Therapy Facts. (2016, February). Retrieved from https://www.lls.org/
[3]Pham, Xuan. (2017). CAR T-Cell Immunotherapy on the Cusp of FDA Approval. Retrieved from https://www.labroots.com/.