Antimicrobial Susceptibility Testing to Detect Antimicrobial Resistance: Laboratory QC Test

Antimicrobial Resistance (AMR) is currently considered a global health concern due to the emergence and spread of multiresistant bacteria not treatable with existing antimicrobial drugs. This resistance develops mainly due to the misuse and overuse of antimicrobials.
The simplicity of microbes allows them to undergo genetic changes that give rise to antimicrobial resistance genes (ARGs). These ARGs equip the microbes with defensive and/or evasive mechanisms against known drugs. Further, they can transfer these genes easily through horizontal gene transfer.
ANTIMICROBIAL SUSCEPTIBILITY TESTING
Empirical therapy, or treatment based on experience, is still considered reasonable in cases where there is no complete medical information or in life-threatening situations such as sepsis. However, with the rise of AMR, precision medicine techniques should be employed to ensure the right antimicrobial therapy for the patient.
Bacterial identification and antimicrobial susceptibility testing (AST) are the most common techniques to determine the correct drug or therapy for the patient. There are different methods for antimicrobial susceptibility testing of the causative pathogen including disk diffusion, gradient strip diffusion, and automated methods. Even with various methods, laboratories conducting the testing should still verify the accuracy and repeatability of the AST method based on the international standard of the Clinical and Laboratory Standards Institute (CLSI).
Quality control for AST of E. coli, N. meningitidis, H. influenzae, and S. pneumoniae
Laboratories should choose which strain to use for Quality Control (QC) testing based on the antimicrobials being regularly used for AST. Strains and corresponding limits are recommended by CLSI as well as other regulatory bodies.
| N. meningitidis: MHA with 5% sheep blood |
Incubate in CO2 incubator with 5% CO2 for 20-24 hours at 35±2°C |
| H. influenzae: Haemophilus test medium (HTM) |
Incubate in CO2 incubator with 5% CO2 for 16-18 hours at 35°C |
| S. pneumoniae: Mueller-Hinton broth |
Incubate in CO2 incubator with 5% CO2 for 20-24 hours at 37°C |
| Negative control: E. coli ATCC 25922 in Mueller-Hinton broth |
Incubate in Laboratory incubators for 16-18 hours at 35°C |

Subculture of the QC strain in the appropriate media. Prepare suspension in the recommended medium.
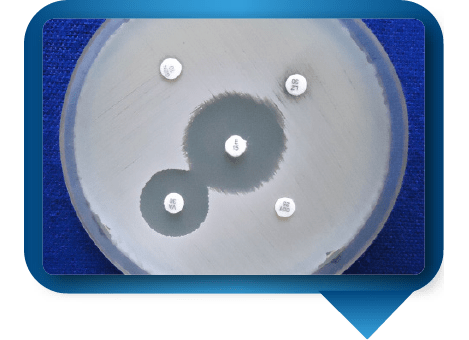
Perform Kirby-Bauer method. Press discs containing specific antibiotics to the plate. Interpret results post-incubation.
Ensure reliability of antimicrobial susceptibility testing
with Esco Lifesciences' cost-effective products.
Read more here: Medical Microbiology
In the Loop: AST to Detect AMR: Laboratory QC Test
References:
[1] Center for Disease Control and Prevention. April 2016. https://www.cdc.gov/meningitis/lab-manual/chpt11-antimicrobial-suscept-testing.html
[2] World Health Organization. October 2020. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance


