Complex cGMP Requirements? Esco LA2 G Series for Pharma Has You Covered

In biopharma manufacturing, the safe handling of liquids and powders is crucial during the quality control process, both for product and user safety. Users rely on controlled environments, typically with biosafety cabinets, to prevent contamination and protect themselves during sensitive tasks.

Yet, regulatory compliance adds another layer of complexity. Biosafety cabinets today must comply with current Good Manufacturing Practices (cGMP). Regulatory frameworks in Europe, such as EU GMP Annex 1, and in countries across Asia, set strict standards for airflow, sterility, and operational performance. For instance, a cGMP-compliant biosafety cabinet must maintain an airflow of 0.45 m/s to ensure both product and operator protection. In addition, auditors will check not only for compliance but also whether these cabinets enable consistent, optimal operation every day.
The Esco LA2 G Series for Pharma is designed to address these exact challenges. With precise airflow control, ISO Class 4 certification for ISO 14644-1:2015 and ISO 14644-14:2016 Cleanroom compatibility, and performance testing in accordance with EN 12459 compliance, this biosafety cabinet gives biopharma manufacturers the confidence that both regulatory requirements and everyday productivity needs are met.
Hence, the new LA2 G-Series for Pharma complies with GMP by incorporating the following design elements:

Full Stainless Steel 304 or 316L Construction for Corrosion Resistant
Because pharmaceutical biosafety cabinets are cleaned daily with strong disinfectants like Spor Klenz (containing 2% peracetic acid), regular materials, even standard stainless steel, can corrode quickly. To address this, this Esco LA2 G-Series for Pharma offers:
- Full Stainless Steel 304 construction, ideal for regular disinfectants.
- Optional upgrade to Stainless Steel 316L, for high-resistance against 316L aggressive cleaning agents.

Viable & Non-Viable Sampling Ports for Continuous Sterility Monitoring
The assurance of safety relies on the ability to effectively monitor it. The Esco LA2 G-Series for Pharma makes this simple and efficient with:
- Viable sampling port for connecting to a microbial air sampler.
- Non-viable sampling cone to connect a particle counter.
Both ports are built into the interior back wall, and their location can be customized. For added convenience, a built-in equipment rack allows users to neatly mount monitoring devices directly on the cabinet stand.
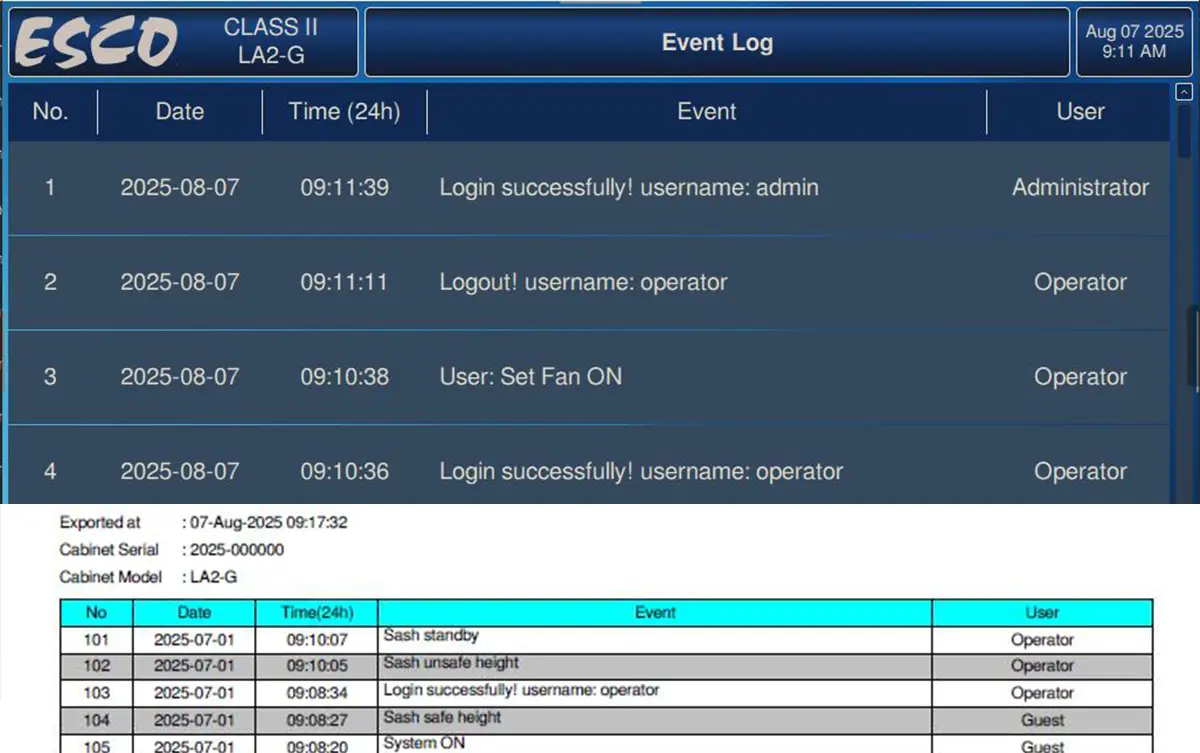
21 CFR Part 11 Compliant Controller
Pharma cGMP facilities require controllers that ensure data logs cannot be altered or erased. To meet this need, the new Esco LA2 G-Series Pharma Biosafety Cabinet is equipped with a built-in 21 CFR Part 11–compliant controller that securely records cabinet usage and operating conditions, ensuring full traceability. This makes your biosafety cabinet fully audit-ready.

Minimal Particle Release to Cleanroom up to ISO Class 4
The Esco LA2 G-Series for Pharma is designed to minimize particle release into the cleanroom (≤0.45 m/s, or 90 fpm), keeping the environment highly controlled for pharmaceutical production. It is ISO Class 4 certified (ISO 14644-1:2015), ensuring very low particle levels for sensitive operations, and meets ISO 14644-14:2016, confirming the cabinet maintains cleanroom integrity. The cabinet is also tested to EN 12469 standards, verifying its airflow and contamination control protect both products and operators reliably.
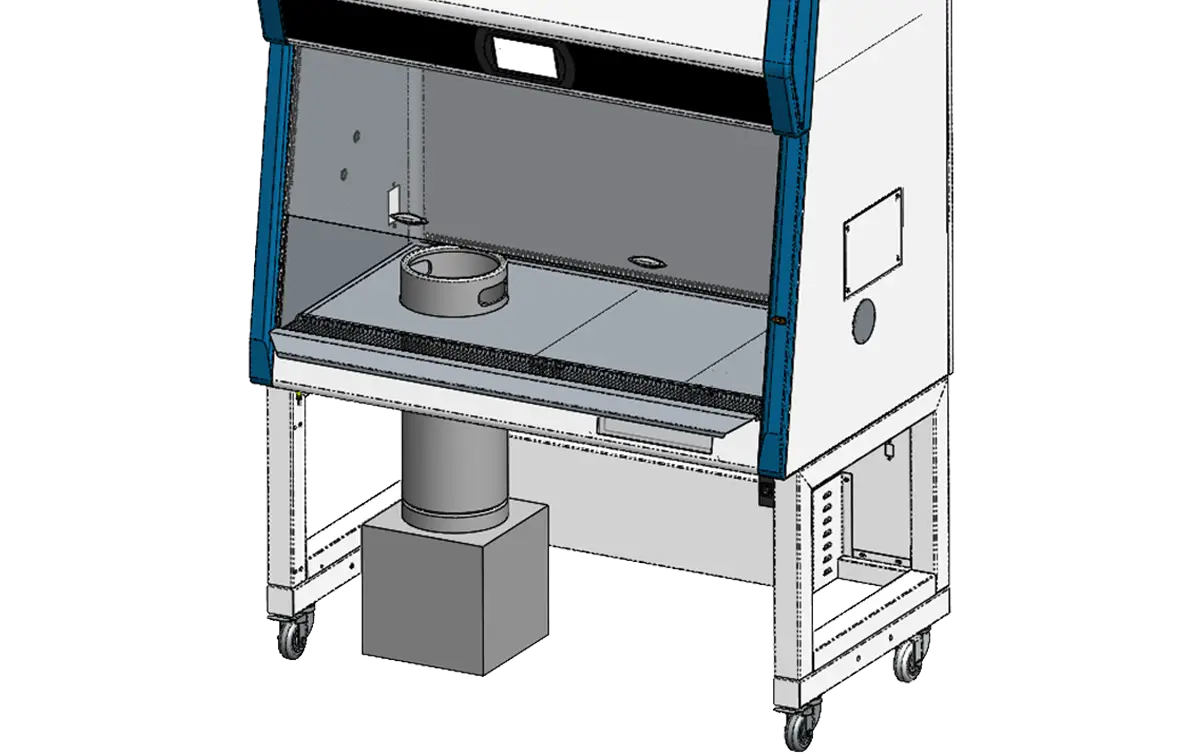
Integrated Bulk Powder Transfer for Efficient Handling in Limited Spaces
Bulk powder transfer is typically performed inside large and costly downflow booths. To offer a more space- and cost-efficient solution, the new Esco LA2 G-Series for Pharma Biosafety Cabinet features an optional cutout on the tray and drain pan.
When paired with a motorized stand, this design allows a drum containing pharmaceutical product to slide easily underneath the cabinet, which can then be lowered until the top of the drum aligns perfectly with the work zone. A built-in guide on the stand ensures precise alignment of the drum with the cutout, facilitating safe and efficient powder transfer.
Delivering Advanced Protection and Compliance for Modern Pharma Labs
In pharmaceutical manufacturing, where quality control is critical and cGMP requirements are increasingly stringent, the new LA2 G-Series for Pharma offers a smart, scalable solution. It is designed to meet the unique demands of the industry, combining premium materials, audit-ready features, and integrated functionality.
Esco’s biosafety cabinets go beyond ordinary lab equipment. They are engineered systems that protect your process, your product, and your people—empowering you to advance global health.

LA2-4G9-S4 and S6 G4
- Single-piece stainless steel 316L internal walls with round corners, easy to clean for GMP
- Full stainless steel 304 or 316L exterior and interior
- Optional viable and non-viable probes
- Built-in Tray Rods for easy cleaning Standard Cable / Tube Port
- 7” touchscreen controller with airflow monitor and self-guide
- Auto-compensating ECM blower, for stable airflow
- 21 CFR Part 11-compliant controller
- cGMP-compliant downflow at 0.45 m/s (90 fpm) for optimum work zone sterility
- Also available in 6-foot size
References
- Abou-El-Enein, M., & Elsanhoury, A. (2020). Sterility testing for cellular therapies: What is the role of cGMP? Frontiers in Bioengineering and Biotechnology, 8, 1–9. https://doi.org/10.3389/fbioe.2020.00761
- Deshpande, A., et al. (2014). Evaluation of a sporicidal peracetic acid/hydrogen peroxide blend for environmental decontamination. Journal of Hospital Infection, 86(4), 271–277. https://doi.org/10.1016/j.jhin.2013.12.015
- International Organization for Standardization. (2015). ISO 14644-1:2015 – Cleanrooms and associated controlled environments – Part 1: Classification of air cleanliness by particle concentration. ISO. https://www.iso.org/standard/53394.html
- International Organization for Standardization. (2025). ISO 14644-5:2025 – Cleanrooms and associated controlled environments – Part 5: Operations. ISO. https://www.iso.org/obp/ui/en/
- National Cancer Institute. (2019). Routine use and disinfection of biological safety cabinets, incubators, shakers, and centrifuges (Biopharmaceutical Development Program Standard Operating Procedure 19102). Author.
- U.S. Food and Drug Administration. (2000). Q7A good manufacturing practice guidance for active pharmaceutical ingredients. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q7a-good-manufacturing-practice-guidance-active-pharmaceutical-ingredients
- U.S. Food and Drug Administration. (2009). Compliance program 7356.002M, chapter 56: Surveillance inspections of protein drug substance manufacturers. https://www.fda.gov/media/75208/download