Strengthening Pharmacy Compounding: Advancing Precision, Traceability, and Safety

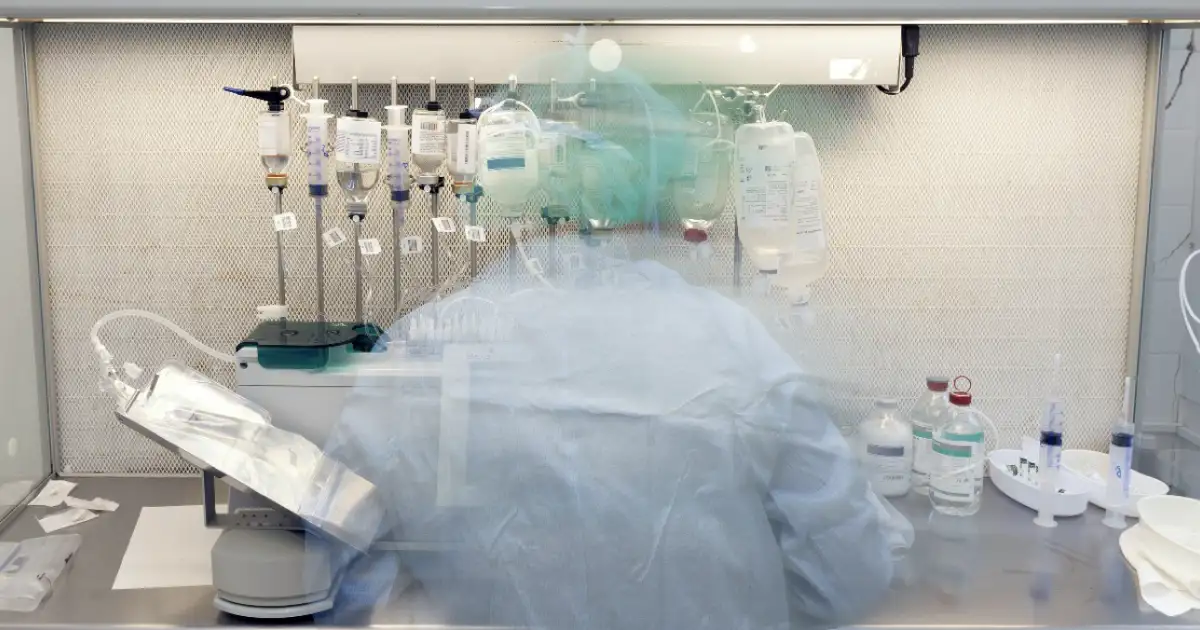
Pharmacy compounding plays an indispensable role in modern healthcare, offering tailored medications for patients whose needs cannot be met by mass-produced pharmaceuticals. Whether it’s adjusting dosages for pediatric care, eliminating allergens, or preparing life-saving intravenous therapies, compounding bridges the gap between standardized treatment and individualized care. In hospitals, oncology units, and specialty clinics, sterile compounding ensures that medications are prepared precisely, aseptically, and according to the highest safety standards. As healthcare continues to advance toward personalization and responsiveness, compounding pharmacies remain a cornerstone of patient-centered care.
As demand increases for personalized medications and regulatory standards become more stringent, pharmacies are continually evolving their practices to improve traceability, minimize human error, and ensure compliance with guidelines like USP <797>, USP <795>, and USP <800>.
This evolution has brought about the integration of technologies—such as camera systems—to enhance verification, training, and documentation. But these tools are not replacements for proper sterile technique; they are extensions of a process rooted in control, safety, and accountability.
Why Camera Systems Are Gaining Ground in Compounding
The role of visual documentation in sterile compounding is growing rapidly. Regulatory agencies and internal quality teams are increasingly calling for verifiable records of how preparations are made—not just written logs, but objective, image-based evidence.
Camera systems now support key areas like:
- Volumetric verification, where the volume drawn into a syringe can be documented before administration, reducing reliance on retrospective methods like pull-back verification.
- Real-time or asynchronous pharmacist oversight, allowing review of compounded products remotely, improving turnaround without compromising safety.
- Training and quality improvement, through recorded sessions that highlight both exemplary and incorrect technique for instructional purposes.
These benefits are particularly valuable in high-throughput environments or multi-site networks where maintaining consistency is critical. The camera, in this context, becomes a verification tool—not a surveillance mechanism, but a partner in promoting quality and accountability.
Balancing Innovation with Aseptic Integrity
While the integration of digital technologies such as camera systems enhances transparency and process control in sterile compounding, it also introduces important considerations related to aseptic technique and environmental integrity. These systems must be carefully evaluated to ensure they do not compromise critical parameters within the ISO Class 5 environment for sterile compounding.
Key factors include:
- Hands-free operability to prevent disruption of sterile technique,
- Strategic placement to avoid interfering with laminar airflow or creating turbulence,
- Ease of disinfection to maintain cleanroom hygiene protocols,
- And most critically, the compatibility with a validated aseptic environment that supports consistent microbiological control.
In this context, the biosafety cabinet (BSC) plays a foundational role. Camera systems and other verification tools depend on a stable, well-controlled environment to function effectively. An appropriately designed BSC ensures:
- Uniform, unidirectional airflow to protect both the product and operator from contamination,
- Optimized lighting conditions that enable high-resolution image capture without glare or shadow,
- Ergonomic and contamination-conscious configurations that accommodate devices while minimizing particulate disruption or surface bioburden.
For facilities advancing toward digital documentation and automation, selecting a BSC is not solely a matter of biological containment. It is a decision that directly influences workflow integration, equipment interoperability, and long-term compliance with compounding standards such as USP <'797>.
The Path Forward: Purposeful Integration in Sterile Compounding
As sterile compounding continues to evolve, innovations like camera-assisted documentation, barcode verification, and automation will become increasingly integral to quality assurance. However, the foundation of safe and effective compounding remains unchanged: meticulous aseptic technique, accurate recordkeeping, and unwavering attention to patient safety.
To support these innovations without compromising the sterile field, technologies must be integrated thoughtfully and within environments specifically engineered for their use. Biosafety cabinets purpose-built for pharmaceutical applications, such as those developed by Esco Lifesciences, provide the controlled conditions necessary for both manual and digital processes to coexist safely.
By aligning high-performance engineering with evolving compounding practices, these systems help pharmacies not only meet today’s regulatory demands but also prepare for the next generation of patient-centered pharmaceutical care.

Designed for Innovation: Esco’s G4 Cabinets
At Esco Lifesciences Group, we recognize the need for equipment that supports modern documentation practices. Our LHG-4BS-F9 and AC2-4E9-NS G4 biosafety cabinets are designed with glass side walls, allowing compounders to mount cameras outside the cabinet—away from disinfectants and harsh cleaning procedures.
Airstream® NS (E-series) G4 Class II Type A2 Biological Safety Cabinet

Features:
- Dimmable LED lighting system
- Centurion touchscreen controller
- Standby height that activates standby mode sustaining ISO Class 3 work zone as the fan runs at half speed
- USB port to relay operational parameters to Building Management System (BMS)
- Remote Modbus
- Default cable port
- Optional 21 CFR Compliance
- E-Series: Multi-piece work tray, glass sidewalls
- Equipped with DC ECM blower for up to 70% energy savings
- Low power consumption
- Available width sizes: 3 ft, 4 ft, 5 ft, and 6 ft
Airstream® Gen 3 Horizontal Laminar Flow Cabinet

Features:
- High-Quality Construction
- Quiet Operation
- ULPA Filterv
- Rocker Switches
- Isocide™ Antimicrobial Powder Coating