Copper (Cu) vs. Silver (Ag): Which Has Better Antimicrobial Properties?

Copper (Cu) and Silver (Ag) are well-studied for their antibacterial properties establishing their potential to eradicate a wide spectrum of infectious pathogens. Mechanism-wise, both are known to effectively inactivate microbes through ionization where copper and silver ions attach to bacteria causing cell wall disruption and eventual bursting of the membranes, but which one propounds a more promising germicidal effect?
 Nanoparticles Antibacterial Mechanism - Esco Scientific-min.png)
Figure 1. Ag Nanoparticles Antibacterial Mechanism (Yin et. al)
Silver’s efficiency as an antibacterial agent has been evident for centuries. In the form of silver nanoparticles (Ag NPs), its application in various biomedical devices has advanced significantly. Silver nanoparticles’ efficacy depends on varying factors: nanoscale size and the large ratio of surface area to volume.
Application-wise, it can be incorporated into various fields such as dentistry (e.g., acrylic resins for dentures, orthodontic treatment adhesive materials, and titanium coating in dental implant treatment), therapeutics (e.g., surgical mesh, wound dressing, artificial joint replacements, and curative catalyzing wound healing), medical imaging, and molecular diagnostics.
 Nanoparticles Antibacterial Mechanism - Esco Scientific-min.png)
Figure 2. Cu Nanoparticles Antibacterial Mechanism (Makvandi et. al)
Similar to silver, copper’s antimicrobial effect has also been known and utilized for ages. In 2600 BC, Egyptians were the first to mention copper’s antimicrobial effects where it is used to purify drinking water and cure chest wounds. Copper was also mentioned in first-century medical writing as an important drug for medical practitioners treating venereal disease and chronic ulcers.
Regardless of the documented benefits, interest in copper was reduced due to the limited effectiveness evidence, and significantly affected by the eventual discovery of antibiotics.
Presently, copper has managed to regain its place in multiple applications backed by several copper compounds possessing antimicrobial abilities such as copper carbonate, copper iodide, and copper thiocyanate. Application varies as some are plied to treat lumber, for ships coatings inhibiting mollusk growth, while there are others that can be found in face creams as well as wound dressings. In the clinical setting, copper has also been proven effective in averse antibiotic-resistant bacteria like MRSA. Its biocidal effect relies on several factors: concentration, exposure time, humidity, and temperature.
Summarily, copper is observed to be effective at varying levels of humidity and temperature, which is due to copper's two ionic states which are Cu+ and Cu2+ compared to silver having one, Ag+. On the other hand, studies show that silver’s antimicrobial property is best if present in a wet environment but at room temperature and nominal humidity of 20%, its antimicrobial activity is almost inexistent. Hence, at the lack of moisture, silver is considered an ineffective biocide.
Table 1. Comparison of Application and Effectivity of Silver and Copper
|
Silver |
Copper |
||
|
Nanoparticle |
Ag NPs |
Cu NPs |
|
|
Use |
-biomedical devices -dentistry (e.g., acrylic resins for dentures, orthodontic treatment adhesive materials, and titanium coating in dental implant treatment) -therapeutics (e.g., surgical mesh, wound dressing, artificial joint replacements, and curative catalyzing wound healing) -medical imaging -molecular diagnostics |
-purify drinking water and cure chest wounds in ancient times -venereal disease and chronic ulcer treatment -for treating lumber -ship coatings for inhibiting mollusk growth -face creams as well as wound dressings -antibiotic-resistant bacteria like MRSA |
|
|
Effectivity in varying environment |
Wet environment | Enhances efficacy | Maintains Efficacy |
| Room temperature | Reduced efficacy | Enhances efficacy | |
| Humid Environment | Reduced efficacy | Maintains Efficacy | |
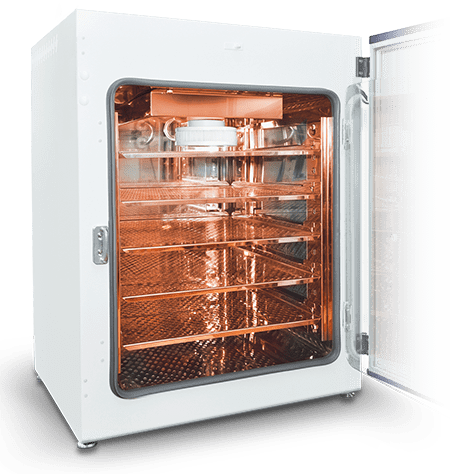
Secure your samples with added antimicrobial feature of Esco CelCulture® CO2 Incubator with Copper Interior Chamber
- 100% Pure Solid Copper Interior Chamber
- FDA-listed, Class II, 510k exempt medical device
- Available in 50 L, 170 L, and 240 L models
Read more: The Lab Cycle: Esco Scientific Quarterly Newsletter - Issue 9, Apr – Jun 2022, Choosing the Right CO₂ Incubator
References:
[1] Arendsen, Linda, et al. “The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology - PMC.” PubMed Central (PMC), www.ncbi.nlm.nih.gov, 1 Oct. 2019, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6730497/.
[2] “Copper or Silver | Which Is the Best Antimicrobial? | Cupron.” Cupron, cupron.com, https://cupron.com/copper-vs-silver-which-is-the-best-antimicrobial/#_edn2. Accessed 16 May 2022.
[3] Eremenko, A. M., et al. “Antibacterial and Antimycotic Activity of Cotton Fabrics, Impregnated with Silver and Binary Silver/Copper Nanoparticles - PMC.” PubMed Central (PMC), www.ncbi.nlm.nih.gov, 1 Jan. 2016, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4717125/
[4] Gaetke, Lisa M., et al. “Copper: Toxicological Relevance and Mechanisms - PMC.” PubMed Central (PMC), www.ncbi.nlm.nih.gov, 1 Nov. 2014, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339675/.
[5] Makvandi, Pooyan, et al. “(PDF) Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry: A Review.” ResearchGate, www.researchgate. net, 25 Sept. 2019, https://www.researchgate.net/publication/335938281
[6] Yin, Iris Xiaoxue, et al. “The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry - PubMed.” PubMed, pubmed.ncbi.nlm. nih.gov, 17 Apr. 2020, https://pubmed.ncbi.nlm.nih.gov/32368040/.